Neural Circuits: Avoiding UV light
Living deep within our oceans, lakes, and ponds are small animals known as zooplankton which typically rise to the surface of the water at night and sink towards the bottom during the day. This synchronised movement helps zooplankton avoid harmful ultraviolet (UV) light and escape diurnal predators that hunt during the day (Malloy et al., 1997).
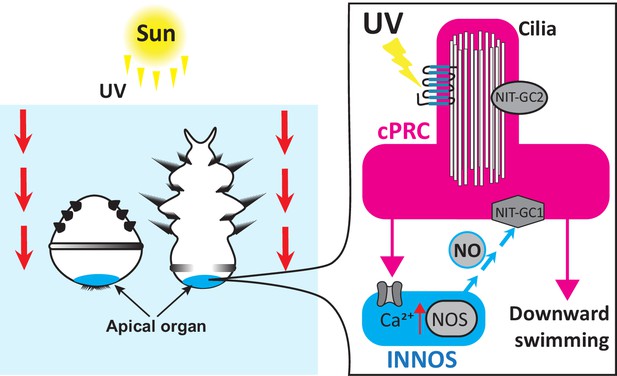
Most marine invertebrates progress through a ciliated larval stage during their life cycle, and this larva will swim freely like zooplankton before settling on the seafloor and transforming into an adult. During this free-swimming stage, the ciliated larvae also avoid UV light, making them a useful model for studying how zooplankton behave. In the larvae of the annelid worm Platynereis dumerilii, this response is controlled by ciliary photoreceptor cells which detect UV wavelengths via a light-sensitive protein known as c-opsin1 (Verasztó et al., 2018; Conzelmann et al., 2013; Arendt et al., 2004). The larvae of other marine invertebrates also use this mechanism to sense UV light (Jékely et al., 2008). However, it was unclear how this sensory input is relayed to the parts of the nervous system that trigger the larvae to swim downwards away from the sun. Now, in eLife, Gáspár Jékely and colleagues – including Kei Jokura as first author – report that P. dumerilii larvae use the gaseous signalling molecule nitric oxide to pass on this information (Jokura et al., 2023).
The team (who are based at the University of Exeter, University of Bristol, Okinawa Institute of Science and Technology and University of Heidelberg) found that the enzyme responsible for generating nitric oxide, nitric oxide synthase (or NOS for short), is expressed in interneurons that reside in the apical organ region, the part of the larva that receives sensory input. Previously collected electron microscopy data from the whole larval body of P. dumerilii was then analysed (Williams et al., 2017), which revealed that these NOS-expressing interneurons lay immediately downstream of UV-sensing ciliary photoreceptor cells.
To further test whether nitric oxide is involved in UV avoidance, Jokura et al. studied P. dumerilii larvae that had been genetically modified so that any nitric oxide produced by these animals emits a fluorescent signal. They found that UV exposure led to higher levels of fluorescence in the part of the larva where the NOS-expressing interneurons project their dendrites and axons. Furthermore, mutant larvae lacking the gene for NOS did not respond as well to UV light, an effect that has been observed previously in mutant larvae that do not have properly working c-opsin1 photoreceptors (Verasztó et al., 2018). These findings confirm the role of nitric oxide in UV-avoidance.
Next, Jokura et al. investigated how nitric oxide signalling affects the activity of ciliary photoreceptor cells using a fluorescent sensor that can detect changes in calcium levels: the more calcium is present, the more active the cell. UV light exposure caused the ciliary photoreceptors to experience two increases in calcium. This biphasic response depended on c-opsin1 and nitric oxide molecules being retrogradely sent from the NOS-expressing interneurons back to the ciliary photoreceptor cells.
Jokura et al. also identified two unconventional nitrate sensing guanylate cyclases (called NIT-GC1 and NIT-GC2) which mediate nitric oxide signalling in the ciliary photoreceptor cells. These proteins are located in different regions of the photoreceptor and may therefore be involved in different intracellular signalling pathways. Experiments with mutant larvae lacking NIT-GC1 confirmed that this protein is necessary for retrograde nitric oxide signalling to ciliary photoreceptor cells. This leads to a delayed and sustained activation of the ciliary photoreceptors, which then drives the circuit during the second increase in calcium. A mathematical model that analysed the dynamics of the neural circuit, and individual cells within it, confirmed that the magnitude of the nitric oxide signal depends on the intensity and duration of the UV stimulus.
In conclusion, Jokura et al. propose that when P. dumerilii larvae are exposed to UV light, this activates ciliary photoreceptors, which, in turn, triggers postsynaptic interneurons to produce nitric oxide (Figure 1). The nitric oxide signal is then sent back to the ciliary photoreceptors, causing them to sustain their activity (even once the stimulus is gone) via an unconventional guanylate cyclase. This late activation inhibits neurons which promote cilia movement. Jokura et al. propose that slowing the beat of certain cilia may rotate the larva so that its head is pointing downwards, causing it to swim away from UV light at the water surface.
The neural circuit that instructs ciliated larvae to avoid UV light.
Two- and three-day-old larvae of the annelid Platynereis dumerilii swim downwards to avoid UV exposure from the sun. The UV light is detected by ciliary photoreceptor cells (cPRCs, pink) which activate interneurons (INNOS, blue) downstream by increasing their calcium (Ca2+) levels. This triggers the enzyme nitrogen oxygen synthase (NOS) to generate the gaseous signalling molecule nitric oxide (NO) which is sent back to the ciliary photoreceptors. Nitric oxide interacts with a nitrate sensing guanylate cyclase (NIT-GC1) which sustains the activity of the ciliary photoreceptors. This signal activates a chain of downstream neurons resulting in the larvae swimming downwards away from UV light at the water surface.
As animals have evolved, their light-response systems have become increasingly sophisticated, especially with the addition of neurons which have further refined this process. Nitric oxide is an ancient signalling molecule that regulates many processes in animals, and its newly discovered role in the ciliated larvae of P. dumerilii may help researchers find missing connections in the light-sensing pathways of other marine invertebrates.
References
Article and author information
Author details
Publication history
- Version of Record published: October 18, 2023 (version 1)
Copyright
© 2023, Sachkova and Modepalli
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 483
- views
-
- 49
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
Primates can recognize objects despite 3D geometric variations such as in-depth rotations. The computational mechanisms that give rise to such invariances are yet to be fully understood. A curious case of partial invariance occurs in the macaque face-patch AL and in fully connected layers of deep convolutional networks in which neurons respond similarly to mirror-symmetric views (e.g. left and right profiles). Why does this tuning develop? Here, we propose a simple learning-driven explanation for mirror-symmetric viewpoint tuning. We show that mirror-symmetric viewpoint tuning for faces emerges in the fully connected layers of convolutional deep neural networks trained on object recognition tasks, even when the training dataset does not include faces. First, using 3D objects rendered from multiple views as test stimuli, we demonstrate that mirror-symmetric viewpoint tuning in convolutional neural network models is not unique to faces: it emerges for multiple object categories with bilateral symmetry. Second, we show why this invariance emerges in the models. Learning to discriminate among bilaterally symmetric object categories induces reflection-equivariant intermediate representations. AL-like mirror-symmetric tuning is achieved when such equivariant responses are spatially pooled by downstream units with sufficiently large receptive fields. These results explain how mirror-symmetric viewpoint tuning can emerge in neural networks, providing a theory of how they might emerge in the primate brain. Our theory predicts that mirror-symmetric viewpoint tuning can emerge as a consequence of exposure to bilaterally symmetric objects beyond the category of faces, and that it can generalize beyond previously experienced object categories.
-
- Neuroscience
The nucleus incertus (NI), a conserved hindbrain structure implicated in the stress response, arousal, and memory, is a major site for production of the neuropeptide relaxin-3. On the basis of goosecoid homeobox 2 (gsc2) expression, we identified a neuronal cluster that lies adjacent to relaxin 3a (rln3a) neurons in the zebrafish analogue of the NI. To delineate the characteristics of the gsc2 and rln3a NI neurons, we used CRISPR/Cas9 targeted integration to drive gene expression specifically in each neuronal group, and found that they differ in their efferent and afferent connectivity, spontaneous activity, and functional properties. gsc2 and rln3a NI neurons have widely divergent projection patterns and innervate distinct subregions of the midbrain interpeduncular nucleus (IPN). Whereas gsc2 neurons are activated more robustly by electric shock, rln3a neurons exhibit spontaneous fluctuations in calcium signaling and regulate locomotor activity. Our findings define heterogeneous neurons in the NI and provide new tools to probe its diverse functions.






